INTRODUCTION
The patients with refractory/ relapsing classical Hodgkin lymphoma (ref/rel cHL) are usually treated with salvage chemotherapy and autologous stem cell transplantation (autoSCT). The ones who fail the treatment with autoSCT are subsequently treated with Brentuximab vedotin (BV) [1]. Use of nivolumab (an immune checkpoint inhibitor) has been approved in the ones who fail the treatments of autoSCT and BV. Because of the high success rate observed with BV and immune checkpoint inhibitors in ref/ rel cHL, they are now often initiated early in management [1].
METHODS
We searched case reports and case series in the PubMed database, from 2014 to 2020, by using the keywords 'Nivolumab' and 'Hodgkin lymphoma' in combination. Forty-eight studies were retrieved. Twenty- eight studies were excluded after reading their abstracts. Subsequently, a study was excluded because it emphasised on the use of Pembrolizumab, and another study was rejected because it was done on a patient of Metastatic squamous cell carcinoma. In the end, eighteen studies were finalized to be included in this review.
RESULTS
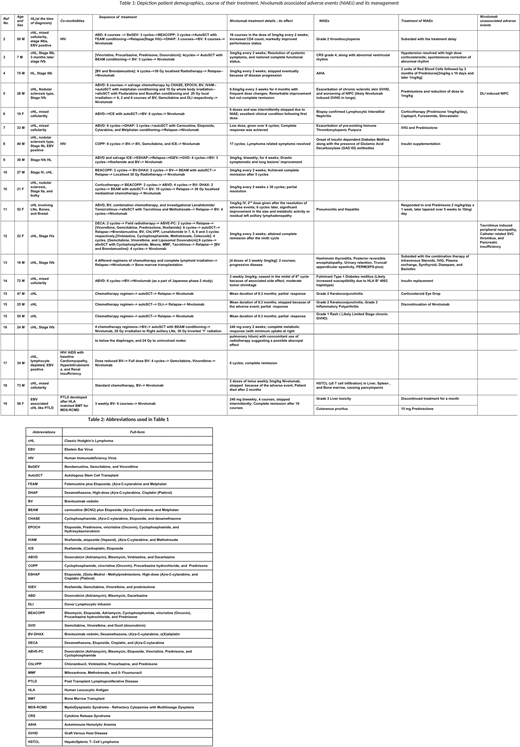
Table 1 highlights the course of treatment in twenty one patients. While six (29%) patients achieved complete resolution of disease, seven (33%) demonstrated marked improvement in their clinical status. Four (19%) went on to accomplish a partial response, whereas one died. Two patients witnessed progressive disease, and one only moderate tumor shrinkage. Nivolumab induced adverse events (NIAEs) were seen in fifteen (71%) patients. Table 2 mentions the abbreviations used in table 1.
DISCUSSION
Nivolumab is a high affinity, a human monoclonal antibody with a half-life approximately 15 days. It received approval by the Federal Drug Administration (FDA) on May 17th, 2016, for patients with rel/ ref cHL [20]. Biomarkers directly correlating with higher progression free survival (PFS) includes a higher degree of 9p24.1 molecular alteration, which is associated with increased expression of PD-L1 and some degree of expression (positive or decreased) of MHC II and CD4+ mediated T-cell response [21]. The adverse reactions that most patients experienced were GVHD involving the skin (most common), pneumonitis, hepatitis, infusion reaction, colitis, and encephalitis or meningitis [22]. These are managed with drug discontinuation, systemic steroids, and tacrolimus. These reactions mostly develop due to imbalanced immune activation and often present as subtle and easily missable features, thus requiring a high degree of suspicion [20].
CONCLUSION
Nivolumab, earlier tried in patients with ref/rel cHL, only is associated with better clinical outcomes. Drug discontinuation can occur as a result of mild-moderate NIAEs. Hence, a high degree of suspicion and prompt management can ensure continued compliance by patients and a better overall prognosis.
REFERENCES
1) Vassilakopoulos TP, et al. Cancers (Basel). 2019
2) Serrao A, et al. Ann Hematol. 2019
3) Foran AE, et al. J Pediatr Hematol Oncol. 2017
4) Tardy MP, et al. Oncol. 2017
5) Onizuka M, et al. Int J Hematol. 2017
6) Oliveira DS et al. Rev Assoc Med Bras (1992). 2019
7) Hwang YY, et. Ann Hematol. 2017
8) Chang E, et al. Clin Lymphoma Myeloma Leuk. 2018
9) Dada R, et al. J Oncol Pharm Pract. 2018
10) de Forceville L, et al. Cancer Radiother. 2019
11) Yared JA, et al. Bone Marrow Transplant. 2016
12) Shad AT, et al. Pediatr Blood Cancer. 2017
13) Tchapyjnikov D, et al. J Immunother. 2017
14) Munakata W, et al. Int J Hematol. 2017
15) Godfrey J,et al. J Immunother Cancer. 2017
16) Wight JC, et al. Leuk Lymphoma. 2018
17) Ding J,et al. Acta Haematol. 2020
18) Ono K, et al. Ann Hematol. 2019
19) Wada F, et al. Ann Hematol. 2020
20) Allen PB, et al. Expert Rev Hematol. 2016
21) Roemer MGM, et al. J Clin Oncol. 2018
22) Bair SM, et al.Oncologist. 2019
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.